Acids and bases
-
LC1.4.4.3C
From the pH value to the pKa value - Digital

-
LC1.4.5.3C
Recording a titration curve - Digital
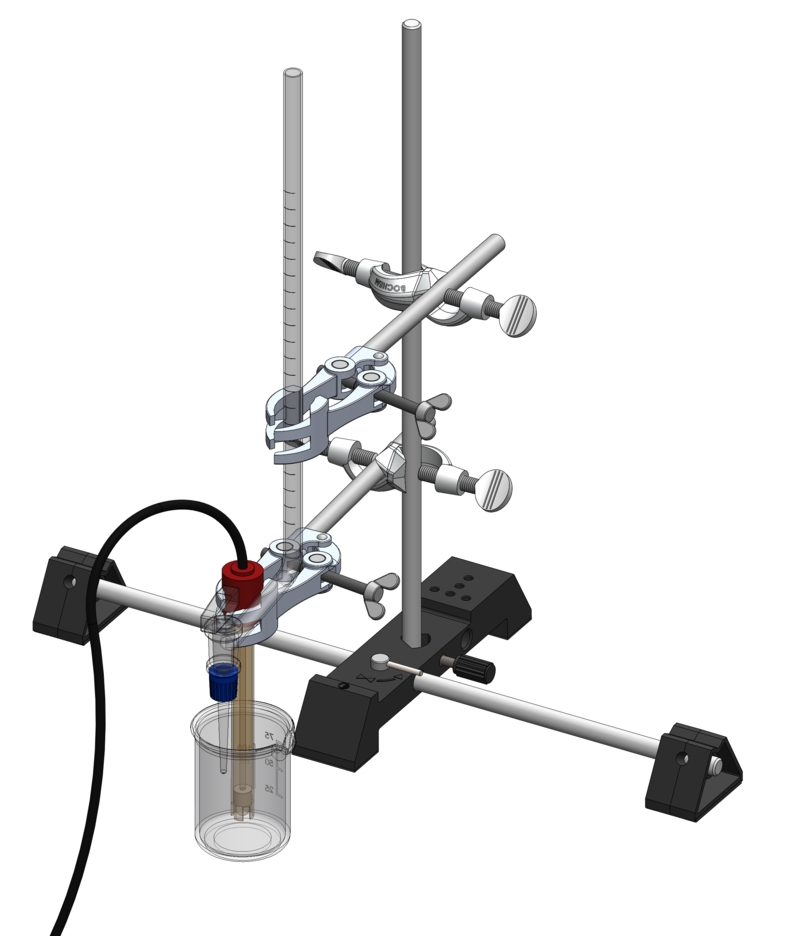
-
LC1.4.6.4
Salt formation through precipitation reactions
-
LC1.4.1.4C
The pH scale - Digital
-
LC1.4.3.4
Reaction of metal oxides with water
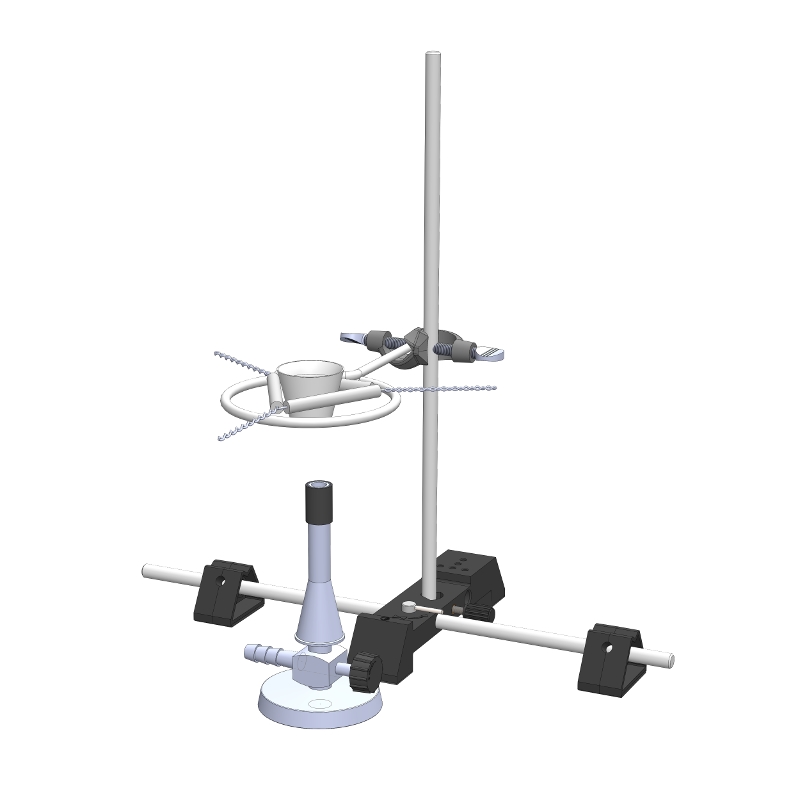
-
LC1.4.5.4
Selecting an indicator for titration

-
LC1.4.1.5C
pH paper versus pH electrode - Digital

-
LC1.4.3.5
Ammonia as a base
-
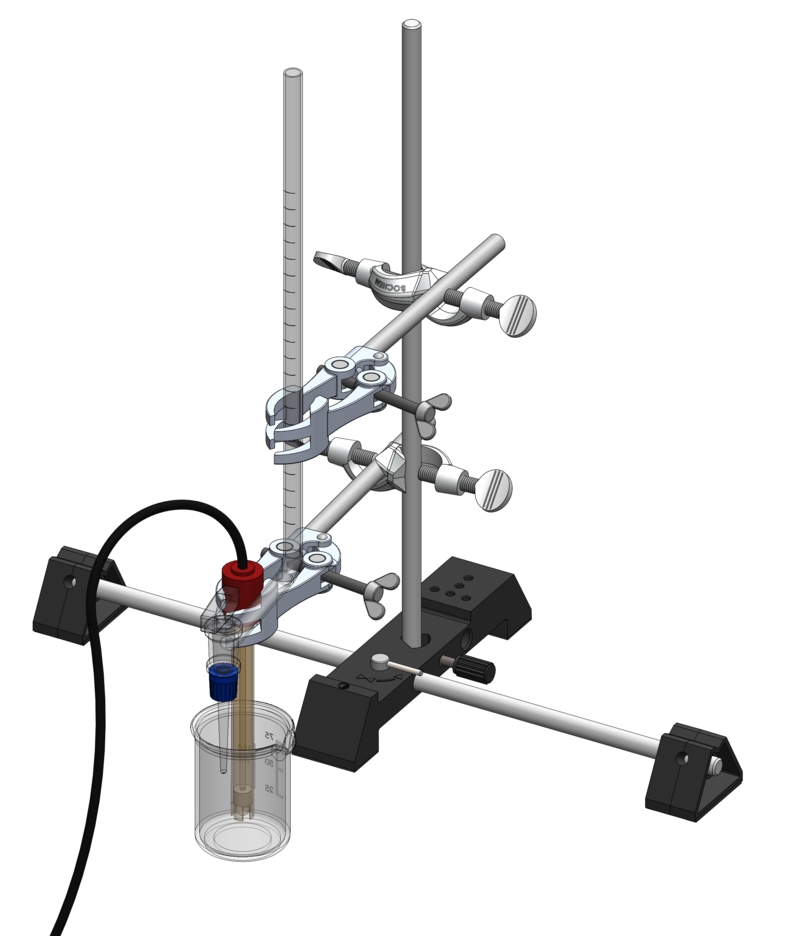
LC1.4.5.4C
Selecting an indicator for titration - Digital
-
LC1.4.1.6
The pH value of everyday chemicals

Print page


