Kinetic theory of gases
-
VP2.5.2.1
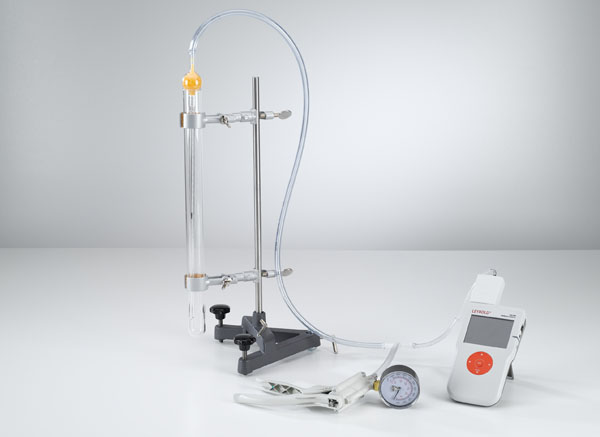
Pressure-dependency of the volume of a gas at a constant temperature (Boyle-Mariotte’s law)
 This product contains dangerous substances!
This product contains dangerous substances!
-
VP2.5.3.1
Determining the adiabatic exponent cp/cV of air after Rüchardt

-
VP2.5.4.1
Joule-Thomson effect

 This product contains dangerous substances!
This product contains dangerous substances!
-
VP2.5.3.2
Determining the adiabatic exponent cp/cV of various gases using the gas elastic resonance apparatus

 This product contains dangerous substances!
This product contains dangerous substances!
-
VP2.5.2.2
Temperature-dependency of the volume of a gas at a constant pressure (Gay-Lussac’s law)

 This product contains dangerous substances!
This product contains dangerous substances!
-
VP2.5.2.3
Temperature-dependency of the pressure of a gas at a constant volume (Amontons’ law)

 This product contains dangerous substances!
This product contains dangerous substances!
Print page
